Abstract
Introduction: Dose adjusted (DA)-EPOCH (etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide) is a first line chemotherapy option for peripheral T-cell lymphomas (PTCLs), but only a minority of patients (pts) are cured. Immune checkpoint blockade (CPB) demonstrated an overall response rate (ORR) of 30% in heavily pre-treated PTCLs in phase I/II studies but rapid or hyperprogression has also been observed in some non-responders. Cytotoxic chemotherapy may be additive to CPB by increasing the expression of neoantigens and therefore overcoming resistance due to weak tumor immunogenicity (Blood Advances. 2021).
Methods: We conducted a phase I trial using a flat dose of the anti-PD1 antibody nivolumab (Nivo) in combination with DA-EPOCH in newly diagnosed PTCLs. Pts were allowed to receive one cycle of chemotherapy prior to enrollment. Pts received Nivo (360 mg) followed by DA-EPOCH every 21 days for 6 or 5 cycles (if prior chemotherapy) in the absence of toxicity or lack of efficacy. For immune related adverse event (irAE), Nivo was held until resolution to grade (G)1 and on ≤10 mg prednisone. For any G3 irAE, Nivo was withheld during the subsequent cycle, and if recurrence, Nivo was permanently discontinued. For protocol defined serious G3 irAEs (e.g., pneumonitis) or G4 irAE, Nivo was omitted from remaining cycles. After completing chemotherapy, pts had the option to proceed with autologous stem cell transplant (ASCT). Responses were assessed by PET/CT after 2 cycles of Nivo + DA-EPOCH and after the last cycle, using RECIL criteria. The primary objective was investigator-assessed ORR to Nivo + DA-EPOCH. Secondary objectives included toxicity, event free survival (EFS), progression free survival (PFS), and overall survival (OS). Selected baseline pt and tumor characteristics, as well as targeted next generation sequencing and multiplex immunohistochemistry of diagnostic tumor tissue were assessed and matched to response, EFS, and PFS in an exploratory analysis.
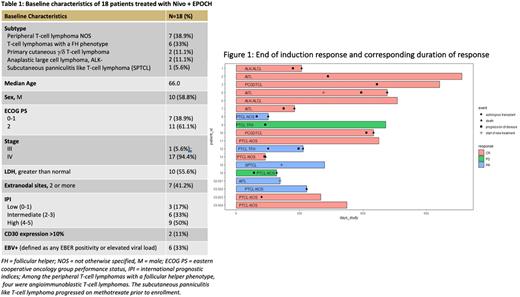
Results: Table 1 displays pt characteristics of the 18 pts enrolled. Nine of 18 pts received treatment prior to enrollment as follows: one cycle of anthracycline based chemotherapy (N=6), oral methotrexate (N=1, SPTCL), and systemic steroids (N=2). The EPOCH dose level ranged from -6 to +3 (median dose level -1). All dose level reductions occurred due to G4 thrombocytopenia. In total, 10% (N=8 of 77) of treatment cycles were delayed >3 days; no delays were due to inadequate hematologic recovery. Immune related AEs of all grades occurred in 78% (N=14) of pts and 39% (N=7) of pts experienced ≥ grade 3 irAEs. 44% (N=8) of pts required discontinuation of Nivo due to irAEs. In the 8 pts whose irAEs resulted in discontinuation of Nivo, the irAE occurred prior to the second or third cycle of Nivo + DA-EPOCH. None of the 6 pts who received a cycle of anthracycline based chemotherapy prior to enrolling on trial experienced an irAE that resulted in dose hold or discontinuation of Nivo, whereas 8 of 12 pts who did not receive a prior cycle of anthracycline based chemotherapy experienced an irAE requiring a dose hold or discontinuation of Nivo. Interim and end of induction ORR were 94% (6 CR, 11 PR; 1 (6%) had SD) and 89%, respectively. We observed 11 CR, 5 PR, and 2 PD at the end of induction. Figure 1 displays response to Nivo + DA-EPOCH and duration of response. 2 pts received consolidation with ASCT. The number of doses of Nivo was significantly associated with improved response (CR vs. PR vs. PD); pts who received more doses of Nivo were more likely to achieve CR (p=0.0066). With a median follow up of 707 days (95% CI: 399-962), median EFS is 373 days (95% CI: 214-616), median PFS is 434 days (95% CI: 225-666), and median OS is 714 days (95% CI: 309-NR). Exploratory analyses, including expression of PD-1 and PDL-1 is ongoing.
Discussion: As expected, in the entire cohort irAEs were common, often leading to discontinuation of CPB. However, no hyperprogression events were observed and dose-limiting irAEs did not occur in pts who received prior chemotherapy. Pts who were able to receive more Nivo had better efficacy outcomes. Given the high-risk characteristics of this cohort, the observed responses in rare subtypes, the encouraging PFS with a long median follow up, and the acceptable toxicity profile, further investigation of chemoimmunotherapeutic treatment strategies is warranted.
Disclosures
Haverkos:Viracta Therapeutics: Consultancy; Bristol Myers Squibb: Research Funding. Zain:Secura Bio: Consultancy, Research Funding; Daichi Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Myeloid: Consultancy, Research Funding; CRSPR: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau; 3M: Current holder of stock options in a privately-held company; Affirmed: Research Funding; Kiyowa Kirin: Speakers Bureau. Kamdar:TG Therapeutics: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; AbbVie: Consultancy; AstraZeneca: Consultancy; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy; ADC Therapeutics: Consultancy; Beigene: Consultancy; ImpactBio: Consultancy; Seagen: Speakers Bureau. Smith:Syros: Research Funding; Kura: Research Funding; AML JV: Research Funding; Argenx: Research Funding. Porcu:Morphosys: Membership on an entity's Board of Directors or advisory committees; DrenBio: Consultancy; Innate Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Teva: Honoraria, Research Funding; ADCT: Membership on an entity's Board of Directors or advisory committees; Ono: Membership on an entity's Board of Directors or advisory committees; Daiichi, Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees; Viracta: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Clinical trial using Nivolumab With Standard of Care Chemotherapy for Peripheral T Cell Lymphomas.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal